 It’s been more than ten years since Caleb began pumping. That’s more than 1,500 Pods, roughly estimating.
It’s been more than ten years since Caleb began pumping. That’s more than 1,500 Pods, roughly estimating.
Caleb was four when we decided to use OmniPod. He didn’t have much say in the matter. Over the years, we’ve talked about other pumps. I thought he might be intrigued by the T Slim because of its slick touch screen. Not so. “Does it have a tube? Yes? No, thanks.” Simple as that.
Traveling on his own and wanting the benefit of closed loop technology that was only available with a tubed pump changed his perspective. Now that we have first hand experience with a tubed pump, I can provide a user comparison of it to a tubeless one.
– The process of changing a site and reservoir is much more involved than filling a Pod and inserting it. There are so many more steps. It’s not difficult – Caleb took over immediately and I don’t even remember what to do – but it’s definitely simpler with OmniPod.
– Being tethered takes some getting used to. Caleb adjusted quickly. Figuring out what to do with the tube with certain clothing, in particular a dress shirt and khakis, was a little perplexing. I sympathize with the ladies wearing dresses.
– This is probably no longer relevant with newer tubed pumps, but the screens and menus of the older model Medtronic pumps – just ugh.
– I now appreciate all the comments about failed Pods. This was never a problem for us. Yes, we had the occasional Pod error, but we didn’t know any differently. It’s what we experienced from the very beginning and we learned to be prepared for it – always carrying extra Pods, insulin and alcohol. No biggie. After using a tubed pump, which almost never errors (we did have a pump go kaput – not fun), I understand how someone switching to OmniPod would find these errors completely unacceptable. Screeching alarms after putting on a staticky winter coat is just not something you experience with a tubed pump. It’s much less risky to leave the house without a site/reservoir change. It’s not risk-free, but the risk is way lower based on our experience.
– Insulin waste. This is another thing I never thought was that big of a deal. The Pod needs a certain amount of insulin to activate. It stops working after 80 hours. Caleb uses less insulin in 80 hours than what is needed to fill a Pod, especially when he started and was using 2 units a day! Yes, I knew insulin was being wasted, and I wasn’t above extracting insulin from a Pod if it failed within the first 24 hours. Caleb thinks it’s a hoot to see how low he can get his reservoir before changing it, even considering letting it go overnight with only 6 units left. Not much waste with a tubed pump.
– Remembering site changes is far easier with the Pod. You have no choice and the alert is relentless. There are times when I ask Caleb – when was your last site change? Silence indicates unknowing. I try to remember to update Nightscout with all his site, CGM and reservoir changes, but I don’t always. I don’t even know the longest time he’s gone with a single site, but it’s over the recommended three days.
– The integrated meter in OmniPod is no small deal. It’s just so convenient and one less thing to possibly forget. Caleb continued to use his PDM as his meter while pumping with Medtronic. It’s worthy to note that the integrated meter will go away with the Dash system.
– There is no comparison regarding the ease of use of OmniPod for sports and similar physical activities. Having to figure out what to do with a tubed pump is just a big pain in the tush. Caleb disconnected for a couple of dance competitions. That didn’t go too well for his bg. He wears it in a belt under his shirt for baseball. That’s less than ideal, for sure. He disconnects to swim – again, his body is not fond of missing any kind of basal insulin and trying to make up for it through microboluses is not something with which we’ve had success. Trying to protect it for paintball – we didn’t bother. He went OmniPod for that one.
– There are more placement options with OmniPod. I know people get creative with weaving their tubes from all spots on their body. It’s so much easier with OmniPod to rotate sites without worrying what to do with the tubing. Caleb sticks to abdomen and legs with Medtronic.
Caleb’s use of Medtronic was planned as temporary – we just wanted a way to help him get through his trip. It seems every time we talk about switching back, it’s deferred to the next site change. For us, OmniPod is the preferred pump choice, but it’s hard to leave the world of Looping once you’ve had a taste of it.



 Animas is discontinuing production and sale of its insulin pumps. Current customers
Animas is discontinuing production and sale of its insulin pumps. Current customers Previously available in Europe and Canada, the Freestyle Libre Flash Glucose Monitoring System was approved by the FDA last week.
Previously available in Europe and Canada, the Freestyle Libre Flash Glucose Monitoring System was approved by the FDA last week. Novo Nordisk released news last week that its faster-acting fast-acting insulin has been approved by the FDA. No need for US citizens to cross the border to Canada to get it anymore!
Novo Nordisk released news last week that its faster-acting fast-acting insulin has been approved by the FDA. No need for US citizens to cross the border to Canada to get it anymore!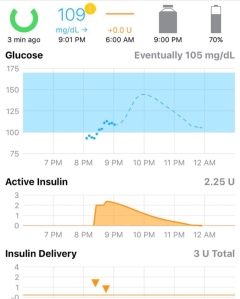 I wasted no time from
I wasted no time from  It was a big news day yesterday for FDA
It was a big news day yesterday for FDA  Another option for managing continuous glucose data is now available in the United States. Abbott’s Freestyle Libre Pro has been FDA approved. This is the Pro version – not the consumer version. This will allow medical professionals to work with their patients to get and analyze data and make therapy decisions. The consumer version has been submitted to the FDA for approval. Hopefully it won’t take too long.
Another option for managing continuous glucose data is now available in the United States. Abbott’s Freestyle Libre Pro has been FDA approved. This is the Pro version – not the consumer version. This will allow medical professionals to work with their patients to get and analyze data and make therapy decisions. The consumer version has been submitted to the FDA for approval. Hopefully it won’t take too long. Full transcript on
Full transcript on  For the full transcript, please go to
For the full transcript, please go to